Category: “Medical Device Manufactures”
- U.S. x-ray market could top $1B by 2020
The U.S. remains the world's largest market for diagnostic x-ray systems, accounting for 29.4% of global revenues in 2013, according to the report. Despite the growth of the x-ray market in China, the U.S. will continue to hold the leading share, with 28% by 2020.
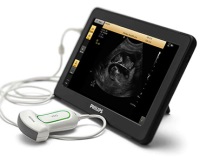
- Philips receives FDA 510(k) clearance for its ultra mobile VISIQ ultrasound system
- FDA Medical Device Guidances Issued in 2014’s Second Quarter
The Food and Drug Administration’s (FDA’s) Center for Devices and Radiological Health (CDRH) has issued a spat of guidance documents this quarter. here are some of the more significant FDA regulations that were issued in the second quarter of 2014. Provided below are brief summaries of several guidance documents that affect manufacturers and marketers of medical devices.
- The current state of the Diagnostic Ultrasound market
- Philips to Reopen Plant to Boost Ailing Healthcare Biz
- Investors put over a Billion in Health IT in Q1 – they think that’s where the sales will be
- Report: Medical Devices produce Healthcare Savings
- M*Modal To Emerge From Ch. 11 With $350M Less Debt
- Analytics is gold – this is where the spend is
Come and listen to a story about a man named Healthcare
A poor mountaineer, barely kept his family fed,
Then one day he was shootin at some food,
And up through the ground came a bubblin crude.
Analytics that is, IT gold, CMS tea.
- No More Business As Usual for MedTech Firms in 2014
Medical Device Manufacturers are seeing revenue from equipment sales decline. Some are entering the business of providing healthcare. Others are finding ways of sharing risk with hospitals that are being paid on outcomes instead of volume. Still others are expanding their offerings in service, consulting, and medical outsourced management. Whatever method used one thing si definite - new revenue models and new business models are required to survive under ACA. In other words innovate or die.
- Carestream’s Digital DRX X-ray Detectors Surpass 9,000 Units
- GE Healthcare faces economic, Obamacare challenges: Immelt
- Boston Scientific’s profits drop like a rock – 97%
- GE Healthcare Sees Slight Profit Increase in Q2
The healthcare division reported revenue of $4.48 billion during the three months ended June 30, compared with $4.49 billion during the same three months in 2013. However, revenues increased from $726 million to $730 million.
- Skanray Technologies Buys X Ray Tube Maker CEI-Italy
- Estimates of Medical Device Spending in the United States
"In view of the conventional wisdom about the role of medical technology in driving up costs, it is surprising that the cost of medical devices has risen little as a share of total national health expenditures. It is also striking that, unlike most other areas of medicine, the prices of medical devices have actually been growing more slowly than both the overall medical or consumer price index."
- Understanding China’s MedTech Business
- Digital mammography costlier, shows no detection-rate advantages: study
- OEMs Benefit from Accelerating M&A Activity in Medtech Outsourcing
Growth and profits are expected from the Medical Device Industry and investors are rushing in. Consolidation is seen as a positive trend.
- Motley Fool says ACA’s Medical Device Tax hasn’t negatively impacted our business
Fool and amuse might be the most appropriate words in Motley Fool's article on ACA's tax on medical equipment manufacturers. The article points out that equipment sold overseas isn't affected and that volumes have not increased as expected to offset this cost. However the Fool insists that the impact has still been marginal. This analysis ignores the flood of companies that have moved their operations overseas, such as GE and Medtronics. The article ignores decreased sales, lower R&D and the tens of thousands of layoffs (Stryker to name just one) that have occurred. Stocks are up but this kind of flawed analysis will be used by politicians to extort more money from an already beleaguered industry.
- Medical Device User Fees Set to Drop in 2015, But Not by Much
- Hospitals want fewer venders but Boston Scientific says they won’t be merging or buying
If scale is what is valued in the healthcare system these days, and hospitals want fewer and fewer vendors, then companies may be compelled to team up, whatever current strategy may be
- Good times for Cerner’s salespeople
- Are Lithium Batteries safe? UL establishes battery consensus standards for medical devices
UL (Underwriters Laboratories) has announced that the U.S. Food and Drug Administration (FDA) has recognized two UL battery safety standards as consensus standards for medical devices incorporating lithium or nickel-based batteries. The two standards are UL 2054 - Standard for Household and Commercial Batteries, and UL 1642 - Standard for Lithium Batteries (Cells).
- Large MedTech conferences is coming up – San Diego Sept 10th and 11th
- Dr. Carlos Nunez, Senior Vice President, Chief Medical Officer, CAREFUSION
- Joni Foy, Ph.D, Office of Device Evaluation Deputy Director, FDA CENTER FOR DEVICES & RADIOLOGICAL HEALTH
- Hovik Gukasyan, PhD, Senior Principal Scientist, PFIZER
- Tom Judd, National Project Director, KAISER PERMANENTE CLINICAL TECHNOLOGY
- Paul Billings MD, PhD, Chief Medical Officer, LIFE TECHNOLOGIES/THERMO FISHER SCIENTIFIC
- Dr. Allison Komiyama, Director of Regulatory Affairs, NEOZENE
- Bruce Buckingham, Professor of Pediatrics & Endocrinology, STANFORD SCHOOL OF MEDICINE
- Monesh Patel, Senior Director of Systems Development - Cardiac Rhythm Disease Management, MEDTRONIC
- Medical Technology Adds $23.6 Billion Annually to Economy
- GMI Ultrasound to Distribute Siemens Products in US
- Varian Medical seeks to revolutionize oncology with developers
- The State of the EHR Market
The EHR market succinctly described – size, market, players.
Five years after ACA’s passage billions of dollars has been poured into the transition to electronic health records. CMS has paid out more than $14.6 billion to hospitals and health systems for the use of EHRs. The EHR market is expected to reach $9.3 billion annually by the end of 2015.
However a once-crowded field of vendors has narrowed significantly. “At the end of 2013, 10 EHR vendors accounted for about 90 percent of the hospital EHR market, based on meaningful use attestation data from CMS: Epic, MEDITECH, CPSI, Cerner, McKesson, Healthland, Siemens, Healthcare Management Systems, Allscripts and NextGen Healthcare.”
Only three, Epic, Cerner and MEDITECH, have more than half of the acute-care EHR market and they are the only companies having experienced market growth in 2013.
- RFID and RTLS (Real Time Locating systems) are becoming big business in Healthcare
RTLS has the potential to save institutions money: Whereas some hospitals used to purchase up to three times the amount of equipment that they needed, just because they had a hard time locating it, effectively implemented RTLS can reduce the need for redundant assets. In 2011, it was reported that 95 percent of organizations that had implemented RTLS had seen a return on investment.